Two researchers from the Artie McFerrin Department of Chemical Engineering at Texas A&M University are making strides in developing the next generation of batteries.
Dr. Perla Balbuena, GPSA Professor, and Dr. Jorge Seminario, Lanatter & Herbert Fox Professor, have been developing protective layers for safer and longer-life batteries,
Balbuena and Seminario’s research is currently in Phase 2 of the Battery 500 project, which is supported by the U.S. Department of Energy (DOE). The continuous support of the DOE has resulted in significant developments in the understanding and design of advanced battery materials that will further the progress of battery technologies for electrical vehicles.
For their research, the pair was awarded a 2020 Texas A&M Engineering Experiment Station's Research Impact Award, which recognizes research that has had an impact, broadly defined as leading to outcomes that extend beyond conventional boundaries, including opening new lines of research, solving a long existing problem or producing tools or products that have become widely adopted in practice by industry and/or government.
The researchers’ work is focused on the mechanisms of electron and ion transport and degradation reactions of electrolyte materials, which have had a profound effect in the development of advanced materials for high-energy density batteries, beyond current lithium-ion (Li-ion) technologies.
The problem with Lithium-metal batteries
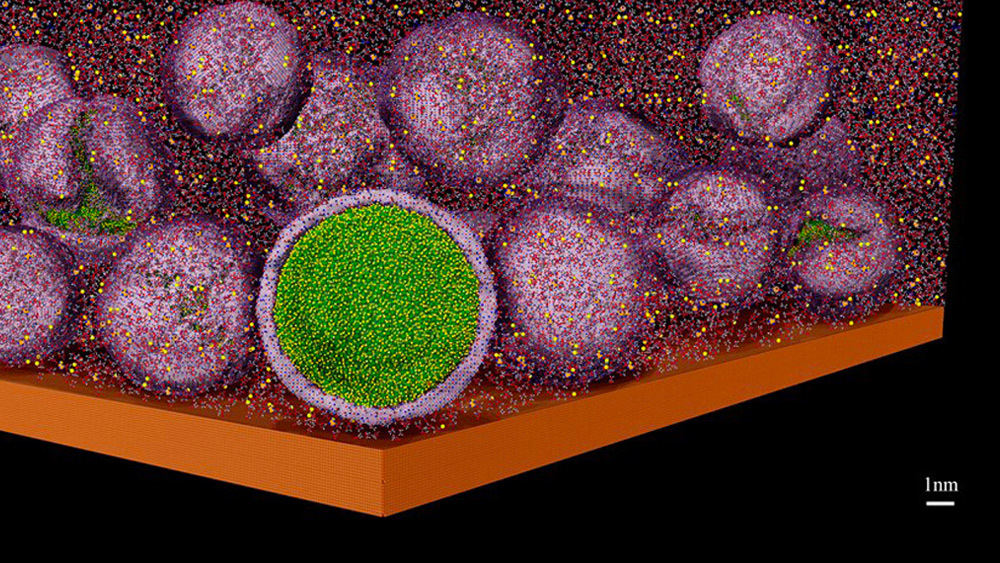
Going beyond Li-ion technologies means that instead of the typical layered intercalation – the movement of lithium ions between the layered materials of the anode and cathode during charge and discharge – materials used in Li-ion batteries, Li-metal is used as one of the electrodes, providing a much higher theoretical capacity. However, stable, long-lived Li-metal batteries are not yet possible due to multiple issues. One of these issues has to do with the interaction between Li-metal and the electrolyte solution – the substance through which the lithium ions pass during charging and discharging. The extreme reactivity of lithium metal causes the formation of microscopic fibers of lithium (called dendrites), which sprout from the electrode and eventually may short circuit the battery.
To prevent or mitigate the dendritic growth, one of the most important factors, yet also one of the least understood, is the formation and properties of solid-electrolyte-interphase (SEI) layers. The continuous passing of electrons between the Li-metal anode and a liquid or solid electrolyte causes the electrolyte layer to degrade. This degradation results in the formation of multicomponent SEI layers on the electrodes. In order to be effective, the SEI films need to act as barriers that stop the transfer of electrons while letting the lithium ions go through and deposit smoothly on the metal surface.
Moving toward stable SEI layers and longer-lasting batteries
The research conducted by Balbuena and Seminario has had a strong impact on the development of protective layers for safer and longer-life batteries. In particular, for the first time, the research team was able to explain the sustained growth of SEI layers at the surface of anodes in Li-ion batteries where the degradation reactions proceed via radical species when the layers thicknesses grow beyond those allowing electron tunneling.
The team was also able to contribute ideas for tuning/designing protective layers by examining nucleation and growth mechanisms of SEI layers derived from the electrolyte material of the battery. Further work discussed the development of a practical device using these ideas and other new concepts related to ionic transport through the interfacial layer. The mechanisms proved by the theoretical and simulation work by Balbuena and Seminario were confirmed by experimental observations and applied to practical devices.
