To develop effective therapeutics against pathogens, scientists need to uncover how they attack host cells first. In that effort, researchers at Texas A&M University have invented a high-throughput cell separation method that can be used in conjunction with droplet microfluidics, a technique whereby tiny drops of fluid containing biological or other cargo can be moved very precisely and at high speeds. Specifically, the researchers successfully isolated pathogens attached to host cells from those that were unattached within a single fluid droplet using an electric field.
“Other than cell separation, most biochemical assays have been successfully converted into droplet microfluidic systems that allow high-throughput testing,” said Dr. Arum Han, professor in the Department of Electrical and Computer Engineering and principal investigator of the project. “We have addressed that gap, and now cell separation can be done in a high-throughput manner within the droplet microfluidic platform. This new system certainly simplifies studying host-pathogen interactions, but it is also very useful for environmental microbiology or drug screening applications.”
The researchers reported their findings in the August issue of the journal Lab on a Chip.
Microfluidic devices consist of networks of micron-sized channels or tubes that allow very controlled movements of fluids. Recently, microfluidics using water-in-oil droplets have gained popularity for a wide range of biotechnological applications. These droplets, which are picoliters (or a million times less than a microliter) in volume, can be used as platforms for carrying out biological reactions or transporting biological materials. Thus, millions of droplets within a single chip facilitate high-throughput experiments, saving not just laboratory space but the cost of chemical reagents and manual labor.
However, biological assays can involve different cell types within a single droplet, which eventually need to be separated for subsequent analyses. This task is extremely challenging in a droplet microfluidic system, said Han.
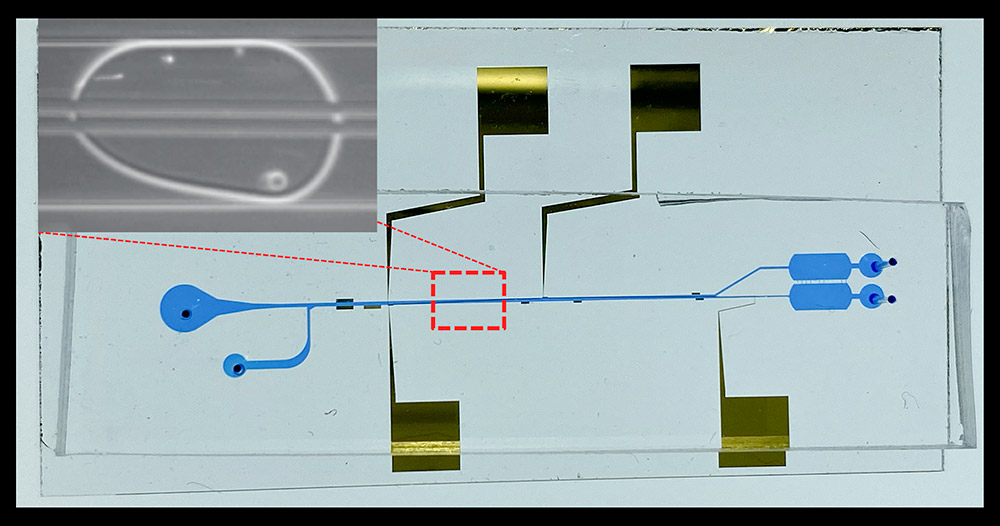
“Getting cell separation within a tiny droplet is extremely difficult because, if you think about it, first, it’s a tiny hundred-micron diameter droplet, and second, within this extremely tiny droplet, multiple cell types are all mixed together,” he said.
To develop the technology needed for cell separation, Han and his team chose a host-pathogen model system consisting of the salmonella bacteria and the human macrophage, a type of immune cell. When both these cell types are introduced within a droplet, some of the bacteria adhere to the macrophage cells. The goal of their experiments was thus to separate the salmonella that attached to the macrophage from the ones that did not.
For cell separation, Han and his team constructed two pairs of electrodes that generated an oscillating electric field in close proximity to the droplet containing the two cell types. Since the bacteria and the host cells have very different shapes, sizes and electrical properties, they found that the electric field produced a different force on each cell type. This force resulted in the movement of one cell type at a time, thereby separating the cells into two different locations within the droplet. To separate the mother droplet into two daughter droplets containing one type of cells, the researchers also made a downstream Y-shaped splitting junction.
“Liquid handling robotic hands can conduct millions of assays but are extremely costly. Droplet microfluidics can do the same in millions of droplets, much faster and much cheaper,” said Han. “We have now integrated cell separation technology into droplet microfluidic systems, allowing the precise manipulation of cells in droplets in a high-throughput manner, which was not possible before.”
This work is funded by the Defense Advanced Research Projects Agency.
