
In a new study, published online in the journal American Chemical Society (ACS) Applied Polymer Materials, scientists at Texas A&M University reported they have designed a hydrogel membrane that may be used to house optical glucose sensing materials, toward building a biosensor for monitoring sugar levels in diabetics.
By incorporating dangling, comb-type molecular chains within a type of hydrogel called poly(N-isopropylacrylamide) or poly NIPAAm for short, they showed that the membrane could prevent leakage of small-sized molecules, like the ones for glucose-sensing, while still allowing glucose to freely diffuse in and out.
When ready for clinical use, the researchers said these membranes could be used to form biosensors that could be easily implanted under the skin of the wrist and might offer a more comfortable alternative to transdermal implants, which sit partially outside the skin. Moreover, unlike transdermal implants that need to be changed every few weeks, this type of subcutaneous implant may only need to be replaced every few months.
“We've done a lot of work on hydrogel materials looking at mechanical properties and foreign body reactions, but our grand goal has always been to use poly NIPAAm membranes to build a subcutaneous glucose biosensor,” said Dr. Melissa Grunlan, professor and holder of the Charles H. and Bettye Barclay Professorship in the Department of Biomedical Engineering. “In this study, we have been able to fine-tune the diffusion properties of these hydrogels that we have previously identified as a promising candidate for building long-term functioning glucose biosensors.”
Poly NIPAAms are a class of organic hydrogels that have a soft texture, like contact lenses. One of their attractive properties is that they can undergo cyclical swelling and deswelling with small temperature fluctuations in the body. Since their surface is dynamically changing with temperature, they deter the attachment of cells and biomolecules. This active, self-cleaning mechanism makes poly NIPAAm hydrogels appealing for implants since they minimize the attack from the immune system.
To use the poly NIPAAm membrane for monitoring blood sugar, it must house enough glucose-sensing molecules or assays. Furthermore, the longevity of the hydrogel also depends on the membrane’s ability to retain these assay molecules without their leaking out.
“Think about the NIPAAm hydrogel like a knitted sweater where the spaces between the meshes are formed by the crossing stitches. Right now, these spaces or windows in the hydrogels are too big, letting the assay molecules go right through,” said Grunlan. “If the assays keep leaching out this way, we're not going to have a long functioning sensor.”
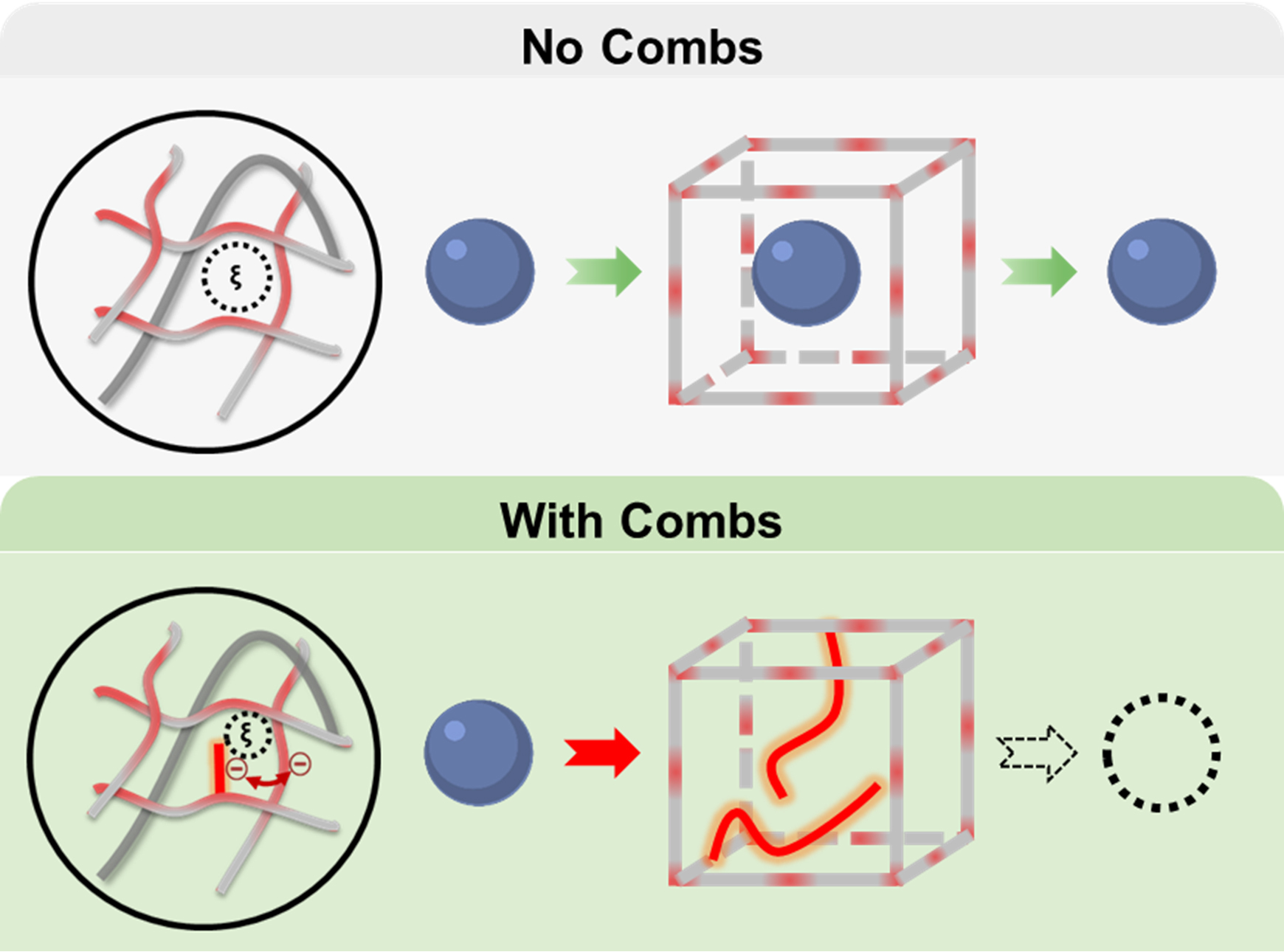
Therefore, Grunlan and her team focused their efforts in fine-tuning the properties of poly NIPAAms to limit the leaking of glucose-sensing molecules while still allowing the glucose to freely diffuse through the hydrogel.
To decrease the size of gaps, the researchers inserted dangling molecules of different charges, lengths and concentrations to the poly NIPAAm hydrogel. When incorporated into the hydrogel, these molecules create comb-shaped barriers, whose teeth are designed to block diffusion of small assay-sized molecules. To test if this comb-like architecture can limit diffusion of glucose sensors, they also put within the hydrogel, fluorescently tagged molecules called dextrans, which served as proxies for glucose-sensing molecules. Next, they placed the hydrogel into water and measured the amount of fluorescence in the water due to the leaking of dextrans from the hydrogel.
The researchers found that when they used a negatively charged molecule called poly(2-acrylamido-2-methyl-1-propanesulfonic acid) or PAMP, the combs prevented the diffusion of dextrans. Furthermore, they also observed that glucose molecules were unhindered in their flow in and out of the hydrogel.
Grunlan noted that now that they have proof-of-concept that their hydrogels can curb leaking of small dextrans, the next step in their research would be to build a biosensor with glucose-sensing molecules contained within the membrane.
“Even though our present study did not involve actual sensing molecules, it very conclusively and precisely shows you what comb architectures can do for hydrogels to limit diffusion,” said Grunlan. “This was a systematic study to show the effectiveness of our approach and the possibility of extending our findings to other areas of research other than glucose sensing for which hydrogels with limited diffusion need to be designed.”
Contributors to this research include Ping Dong and Bradley Schott from the biomedical engineering department and Dr. Kristen Means, a former student from the Department of Materials Science and Engineering at Texas A&M and current post-doctoral researcher at Rice University.
This research is funded by the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation, the National Science Foundation’s Engineering Research Center for Precise Advanced Technologies and Health Systems (PATHS-UP) and the National Science Foundation Graduate Research Fellowship Program.
