
A typical nuclear reactor uses only a small fraction of its fuel rod to produce power before the energy-generating reaction naturally terminates. What is left behind is an assortment of radioactive elements, including unused fuel, that are disposed of as nuclear waste in the United States. Although certain elements recycled from waste can be used for powering newer generations of nuclear reactors, extracting leftover fuel in a way that prevents possible misuse is an ongoing challenge.
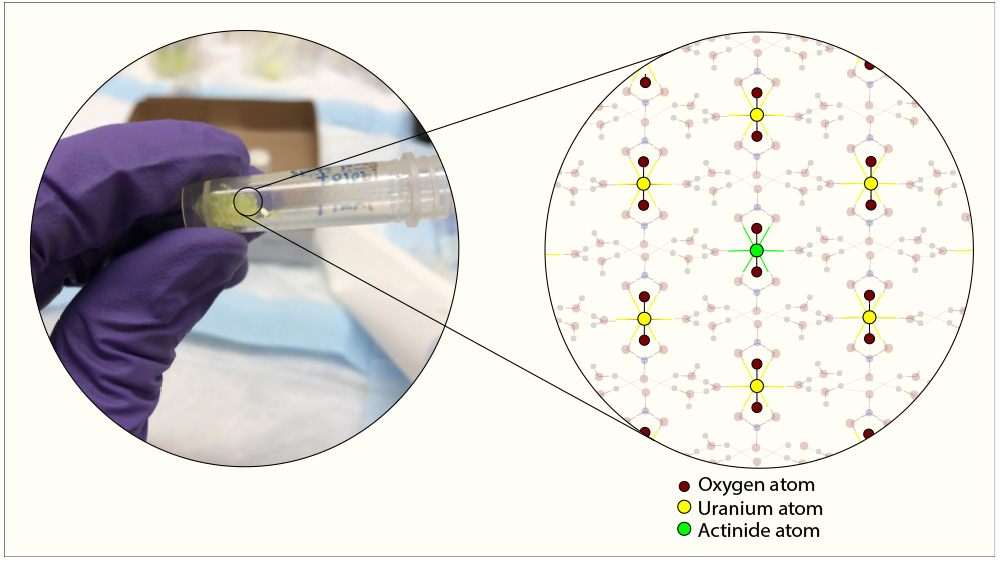
Texas A&M Engineering researchers have devised a simple, proliferation-resistant approach for separating out different components of nuclear waste. The prescribed one-step chemical reaction, described in the February issue of the journal Industrial & Engineering Chemistry Research, resulted in the formation of crystals containing all of the leftover nuclear fuel elements distributed uniformly.
The simplicity of their recycling approach also makes the translation from lab bench to industry feasible.
“Our recycling strategy can be easily integrated into a chemical flow sheet for industrial-scale implementation,” said Dr. Jonathan Burns, research scientist in the Texas A&M Engineering Experiment Station’s Nuclear Engineering and Science Center. “In other words, the reaction can be repeated multiple times to maximize fuel recovery yield and further reduce radioactive nuclear waste.”
This simplified, single-step process is also proliferation-resistant since plutonium is not isolated but incorporated within the uranium crystals.
“In addition to addressing the fuel recycling problem and reducing proliferation risk,” said Burns, “our strategy will drastically reduce nuclear waste to just the fission products whose radioactivity is hundreds rather than hundreds of thousands of years.”
Energy production in nuclear reactors
The basis of energy production in nuclear reactors is thermonuclear fission. In this reaction, a heavy nucleus, usually uranium, when hit by subatomic particles called neutrons, becomes unstable and tears apart into smaller, lighter elements. However, uranium can absorb neutrons and get progressively heavier to form elements like neptunium, plutonium and americium, before once again splitting and releasing energy.
Over time, these fission reactions lead to a buildup of lighter elements in the nuclear reactor. But roughly half of these fission products are deemed neutron poisons—they also absorb neutrons just like used nuclear fuel, leaving fewer for the fission reaction, eventually bringing the energy production to a halt.
Hence, used fuel rods contain fission products, leftover uranium and small quantities of plutonium, neptunium and americium. Currently, these items are collectively considered nuclear waste in the United States and are destined to be stowed away in underground repositories because of their high radioactivity.
