In the perennial clash between man and microbe, ultraviolet light has emerged as one of man’s powerful tools against many pathogens. Although ultraviolet light can wipe out several germs, the exact mechanisms that orchestrate the radiation’s damaging action have long been elusive.
In the September issue of PNAS, Texas A&M University researchers have reported that ultraviolet radiation creates holes in the microbes’ outer protective sheath by dislodging tryptophan — a molecule that is an important component of the bacteria’s outer covering. The researchers said that these holes provide gateways for ultraviolet radiation to go into the bacteria and disrupt its DNA, which then stops the microbes from replicating.
“Our study provides the science behind the germicidal action of ultraviolet light,” said Dr. Peter Rentzepis, TEES Eminent Professor in the Department of Electrical and Computer Engineering and a member of the National Academy of Sciences. “We’d like to use this knowledge to develop better ways to monitor bacteria inactivation in various settings, including the food industry and health care.”
Ultraviolet light is a highly energetic beam of radiation that has been harnessed for a variety of applications ranging from food contamination prevention to infection control. Although ultraviolet radiation has been used for over five decades to kill bacteria, the means by which it enters microorganisms and then accesses their genetic material has not been clear.
To reach the interior of the bacterium, where the DNA-containing nucleus is located, ultraviolet radiation must pass through an outer layer surrounding the microbe, called the cell membrane. Attached to this membrane are tryptophan molecules. Although tryptophan is popularly known for its sleep-promoting effects in humans, in bacteria, tryptophan anchors proteins made within the microbes onto the cell membrane. Consequently, the bacterial outer covering is studded with tryptophan molecules.
Also, unlike most other biological molecules, tryptophan interacts with ultraviolet light. When hit by ultraviolet light, tryptophan molecules absorb the radiation and get energized, and when they lose this absorbed energy, they reemit a much weaker ultraviolet light, dubbed fluorescent light. Rentzepis and his team investigated if these ultraviolet light-tryptophan interactions played a role in killing bacteria.
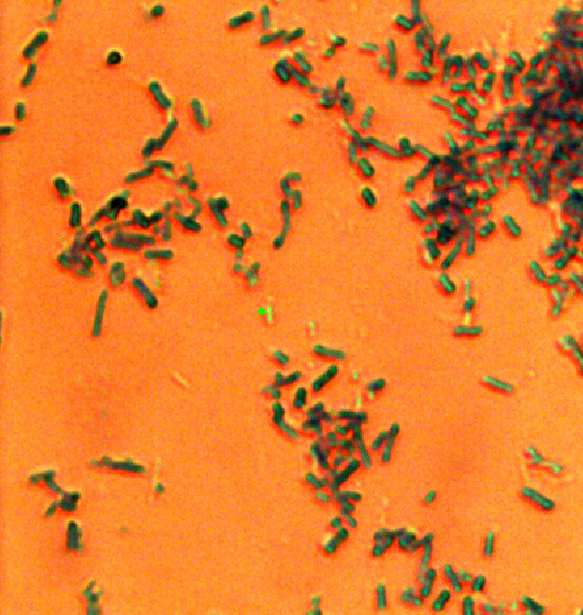
For their experiments, the Texas A&M team looked at the fluorescent light emitted by tryptophan molecules in Escherichia coli and Bacillus subtilis bacteria after shining a beam of ultraviolet radiation on them. As expected, they found that at the end of radiation, which typically lasted several minutes, the fluorescent light emitted by the tryptophan molecules was drastically reduced, indicating that the radiation was killing the bacteria. However, to their surprise, this decreased fluorescent light came after an initial increase, immediately after the radiation was turned on.
“The surge of the emitted ultraviolet light just after the radiation onset made us suspect that changes happening to tryptophan molecules before they are ultimately destroyed by ultraviolet light may be involved in how radiation gets into the bacteria,” said Rentzepis.
Past studies have shown that when hit by ultraviolet light, protein molecules that are normally wound up in complicated shapes unfold and, as a result, expose tryptophan. Rentzepis’ research group’s findings suggest that in bacteria, ultraviolet light might unfold membrane proteins and detach tryptophan molecules, which may then cause the initial increase in the emitted light signal. With tryptophan plucked out of the cell membrane, the space left behind forms gaping holes for the ultraviolet light to enter and damage DNA, he said.
While the general profile of emitted light is similar for different strains of bacteria, Rentzepis noted that there are subtle differences that are unique to each species and keeping track of these differences might help in identifying and cataloging different species of bacteria.
Rentzepis and his team have also developed and patented the technology for a handheld device that can collect emitted light from bacteria during irradiation. “Bacterial invasion can happen in different contexts, from food processing centers to operation theaters,” he said. “But we now have both the science and technology to address these real-world problems since we have a grip on the biological basis behind ultraviolet-induced bacteria inactivation and an instrument that can, within minutes and in situ, determine how many bacteria are dead after radiation.”
Other contributors to the research include Dr. Runze Li and Dinesh Dhankhar, Anushka Nagpal and Arjun Krishnamoorthi from the Texas A&M Department of Department of Electrical and Computer Engineering; Dr. Maria King from Texas A&M Biological and Agricultural Engineering; Dr. Jie Chen from Shanghai Jiao Tong University’s Center for Ultrafast Science and Technology and Dr. Thomas C. Cesario from University of California at Irvine’s School of Medicine.
This work was supported, in part, by the Welch Foundation, the Air Force Office of Scientific Research and the Texas A&M Engineering Experiment Station funds.
